BULLSCRAPS, 2BIG 2FAIL , GANG-BANKSTERS T-Shirt
15 years ago
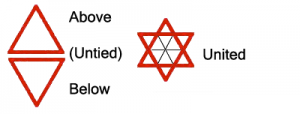
The ancient Hermetic axiom, from about 2,300 years ago, suggests, "As is the inner, so is the outer; as is the great, so is the small; as it is above, so it is below; there is but One Life and Law; and He, that is worth it, is One. Nothing is inner, nothing is outer; nothing is great, nothing is small; nothing is high, nothing is low; in the Divine Economy."

No comments:
Post a Comment